FDA Releases the Food Traceability Final Rule
go.ncsu.edu/readext?904575
en Español / em Português
El inglés es el idioma de control de esta página. En la medida en que haya algún conflicto entre la traducción al inglés y la traducción, el inglés prevalece.
Al hacer clic en el enlace de traducción se activa un servicio de traducción gratuito para convertir la página al español. Al igual que con cualquier traducción por Internet, la conversión no es sensible al contexto y puede que no traduzca el texto en su significado original. NC State Extension no garantiza la exactitud del texto traducido. Por favor, tenga en cuenta que algunas aplicaciones y/o servicios pueden no funcionar como se espera cuando se traducen.
Português
Inglês é o idioma de controle desta página. Na medida que haja algum conflito entre o texto original em Inglês e a tradução, o Inglês prevalece.
Ao clicar no link de tradução, um serviço gratuito de tradução será ativado para converter a página para o Português. Como em qualquer tradução pela internet, a conversão não é sensivel ao contexto e pode não ocorrer a tradução para o significado orginal. O serviço de Extensão da Carolina do Norte (NC State Extension) não garante a exatidão do texto traduzido. Por favor, observe que algumas funções ou serviços podem não funcionar como esperado após a tradução.
English
English is the controlling language of this page. To the extent there is any conflict between the English text and the translation, English controls.
Clicking on the translation link activates a free translation service to convert the page to Spanish. As with any Internet translation, the conversion is not context-sensitive and may not translate the text to its original meaning. NC State Extension does not guarantee the accuracy of the translated text. Please note that some applications and/or services may not function as expected when translated.
Collapse ▲The Food & Drug Administration released the Food Traceability Final Rule on November 17, 2022. The Rule requires covered companies to maintain records for foods on the Food Traceability List (FTL) in order to support more efficient and accurate traceability of potentially contaminated food. Benefits of this new Rule will include: averted foodborne illnesses, reduced food waste, avoiding costs of overly-broad recalls, and improved supply chain management and inventory control.
The FDA hosted a recorded webinar where traceability experts, regulatory officials and subject matter experts convened to cover the following topics:
- Overview of the Final Traceability Rule
- Exemptions to the Rule
- Food Traceability List
- Clarification Question Sessions
- Rule Requirements, including:
- Traceability Plans
- Assigning Traceability Lot Codes
- Critical Tracking Events and Key Data Elements
- Electronic Sortable Spreadsheet
- Records Maintenance and Availability
- Compliance Dates
Food Traceability List
Foods subject to the final rule requirements appear on the Food Traceability List and also include foods that contain listed foods as ingredients (provided the listed food remains in the same form in which it appears in the FTL, for example: fresh).
The Food Traceability List is anticipated to be updated approximately every 5 years.
| Cheese (made from pasteurized milk), fresh soft, soft unripened, soft ripened or semi-soft | Cheese (made from unpasteurized milk), other than hard cheese | Crustaceans (fresh and frozen) | Cucumbers (fresh) |
| Finfish (fresh and frozen) histamine-producing species | Finfish (fresh and frozen) species potentially contaminated with ciguatoxin | Finfish (fresh and frozen) species not associated with histamine or ciguatoxin | Fruits (fresh-cut) |
| Herbs (fresh) | Leafy greens (fresh) | Leafy greens (fresh-cut) | Melons (fresh) |
| Molluscan shellfish, bivalves (fresh and frozen) | Nut butters | Peppers (fresh) | Shell eggs |
| Smoked finfish (refrigerated and frozen) | Sprouts (fresh) | Tomatoes (fresh) | Tropical tree fruits (fresh) |
| Ready-to-eat deli salads (fresh and frozen) | Vegetables (fresh-cut) |
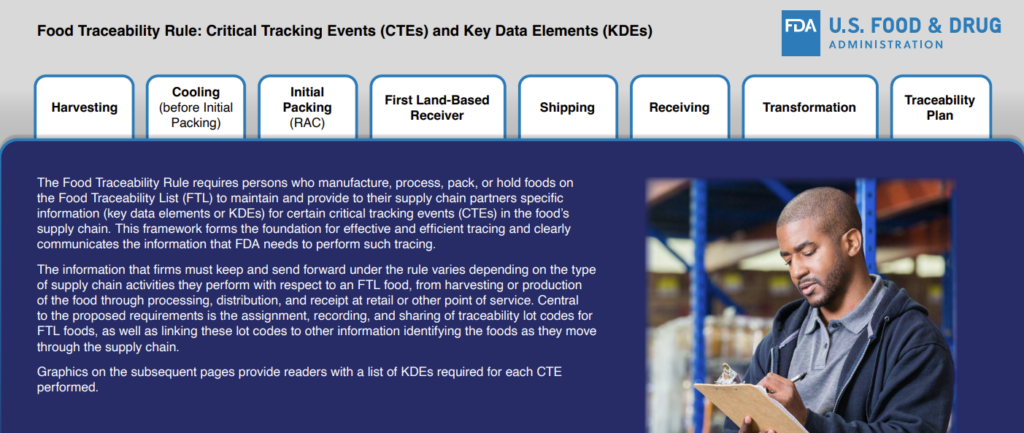
Critical Tracking Events and Key Data Elements
The Rule requires companies who manufacture, process, pack, or hold foods on the FTL to maintain and provide their supply-chain partners with information, or key data elements (KDEs), for certain critical tracking events (CTEs). The type of information that must be kept and forwarded will vary depending on the type of activities the company performs.
The FDA has created a document to help companies understand what KDEs are required for each CTE performed.
Exemptions to the Food Traceability Rule
Exemptions from the Food Traceability Rule are allowed for some companies due to their size, operations, type of processing and other food safety considerations.
The FDA has created an Exemption Tool that can help companies step-by-step through the exemption process.
Food Traceability Compliance Date:

Compliance date applies to all firms that manufacture, process, pack or hold foods on the Food Traceability List. The FDA plans on an “educate before and while we regulate” policy as firms move towards total implementation of the Traceability Rule.